Chronic Myelogenous Leukemia (CML)
-
CML patients often present late. Some have been told there is no treatment or have been treated with Hydroxyurea or splenectomy. Imatinib is the treatment of choice. Prognosis best if Imatinib started early in course of disease. We are the only provider of free Imatinib in Cambodia (gifted by Novartis through GIPAP – “Glivec International Patient Assistance Program”). Customs process is difficult and often delayed. Patients can be seen without the usual referral form. One clinic doctor is registered as our GIPAP physician.
Background/Cultural Points:
Section titled “Background/Cultural Points:” -
Contacts:
Section titled “Contacts:”- Mary Kluck – MMC GIPAP Physician
- Lim Nyda/Registration – MMC GIPAP Administration
- GIPAP Registration – Email MMC GIPAP Physician to forward to GIPAP; typically can be approved in 24 hours.
- GIPAP Information – www.themaxfoundation.org
-
Diagnosis & Lab:
Section titled “Diagnosis & Lab:”- CBC ~diagnostic (beware machine differentials - may not detect precursor cells)
- GIPAP require bone marrow examination for either BCR/ABL OR Philadelphia chromosome (bone marrow histology not required).
- When positive result known and consent form signed, GIPAP physician can apply by email; approval is usually given overnight and patient can start Imatinib next day.
-
Management/Education/Treatment:
Section titled “Management/Education/Treatment:”- Enroll Patient in GIPAP (via Email):
- Patient agrees not to give the drug to anyone else and to return the empty packaging.
- GIPAP agrees to supply drug (only) at no cost to patients.
- Starting Imatinib:
- standard adult dose is 400mg daily (600mg if accelerated or blast)
- take with food or large glass of liquid (if 600mg or more, give divided dose bid)
- minor side-effects common but usually settle (tiredness, peripheral edema, joint pains, minor rash)
- watch for neutropenia and thrombocytopenia (can occur at any time during therapy, but generally within 2-4 weeks of starting Imatinib) and hepatotoxicity
- Managing myelosuppression: aim not to cease Imatinib as may lead to later resistance; however better to cease briefly than to decrease dose.
- chronic phase CML
- stop Imatinib for 1-2 wks if neutrophils <1000 or platelets <50
- if low counts recur, the dose can be reduced (≮ 300mg for adults)
- accelerated phase CML or blast crisis
- even more important to continue the Imatinib if possible
- stop Imatinib if neutrophils stay lower than 500 or platelets <10
- reduce dose if the low neutrophil count due to Glivec (and not CML)
- Women must avoid pregnancy (Imatinib is Category D)
- Enroll Patient in GIPAP (via Email):
-
Follow-Up:
Section titled “Follow-Up:”- Review weekly at first, then monthly (CBC monthly, SGOT/SGPT and Creatinine 2- monthly); 2-monthly if stable long-term.
- Treatment is continued indefinitely.
-
Common Errors & Other Pointers:
Section titled “Common Errors & Other Pointers:”-
Define (and re-define as time goes on) the phase of the disease because it affects treatment plan:
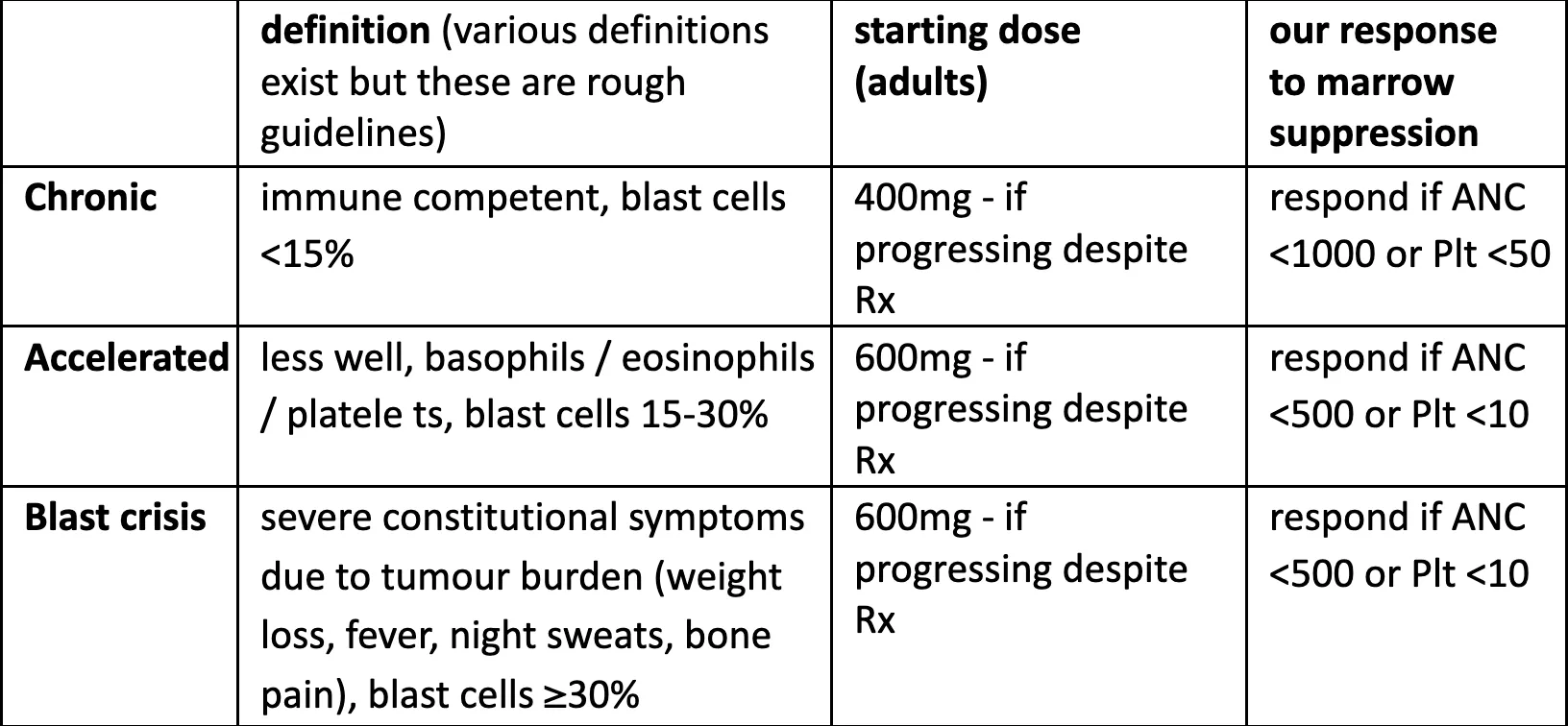
-
Remember that some labs do only machine differentials (i.e. the staff do not look at blood films, they just print a report from a machine count). Machines may count precursor cells as lymphocytes or other normal cells. So if the patient has symptoms or signs of escalation but normal-looking CBC, get CBC and blood film at Pasteur.
-
Try not to stop Glivec. Continuous use from an early stage of the disease gives the best chance of long-term remission. The hematologists tolerate much lower ANC and platelet counts than we like (lower than suggested in UpToDate too). Infection is unlikely with ANC above 500.
-
Chronic phase: stop Glivec 1-2 weeks if ANC <1000 or platelets <50
-
Accelerated phase or blast crisis: stop Glivec 1-2 weeks if ANC <500 or platelets <10
-
If concerned re marrow suppression, stop Glivec briefly. Stopping is better than lowering the dose. Re-try at the same dose. If fails at the same dose a second time, consider a lower dose for a while (but ≮ 300mg for adults).
-
Most marrow suppression occurs in the first 1-2 months but it can occur later too. If on less than the standard dose for the phase, it’s usually worth trying the higher dose again later – often will tolerate it and gives better chance of long-term control.
-
GIPAP will not approve a dose of 200mg for an adult. They believe it is probably ineffective and putting the patient at increased risk of resistance which will make the drug totally ineffective for the patient in the long-term. Much better to stop altogether.
-
Success is not just having a patient with few symptoms and a normal-looking CBC (although I agree that does warm one’s heart!). We are also trying to suppress the disease for the very long-term so we need to stick to doses and schedules that have been proven to do that.
-
Remember effective contraception for female patients. Glivec is Category D.
-
- MORE THOROUGH REFERENCE MATERIALS AVAILABLE IN MMC CLINICAL CARE FILES (MMC LIBRARY)